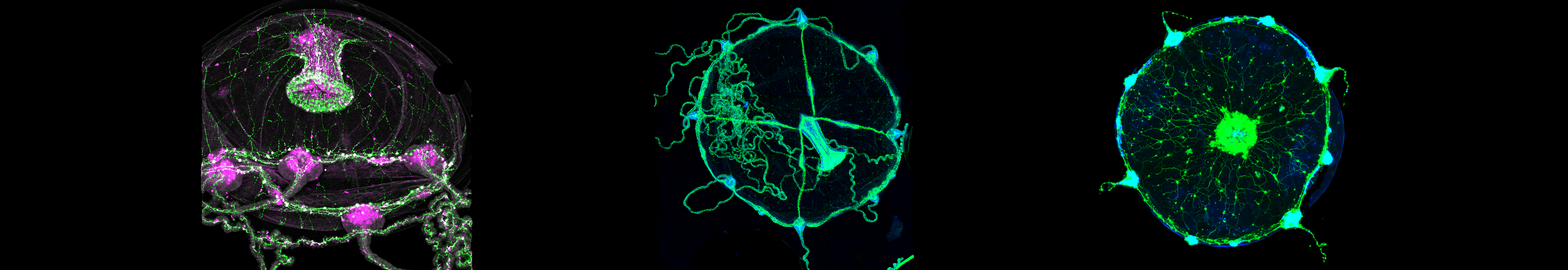
Jellyfish and Polyps Offer New Insight Into How Groups of Neurons Coordinate Behavior
Jellyfish, anemones and coral polyps, known collectively as cnidarians, have captured the imaginations of scientists across biological disciplines for centuries. Their radial symmetries and graceful, fluid movements lend them an undeniable appeal, but it’s their peculiar nervous systems that have drawn recent attention from neuroscientists.
Unlike in most animals, whose neurons are gathered into bundles of nerves and larger structures like brains and ganglia, cnidarian neurons are distributed through their tissues in structures called nerve nets. This diffuse organization makes it possible to observe neural activity from many neurons simultaneously: Because neurons are spread in a thin layer, no neuron blocks an observer’s view of another. That means researchers can use techniques like calcium imaging to potentially capture the activity of a cnidarian’s entire nervous system, rather than a subset of neurons in the dense tangle of a mouse brain, for example.
Neuroscientists are leveraging the accessibility of nerve nets to more deeply explore the properties of neural ensembles, groups of neurons that fire in a correlated fashion. Ensembles are a fundamental feature of the brain; they offer a simple example of functional structure in an animal’s nervous system and have become a popular target for systems neuroscientists because they combine population coding (how neural activity encodes information in populations of cells) and connectivity (how connections among neurons relate to population activity). Understanding how these groups form, how they coordinate patterns of neural activity, and how they drive behavior may reveal organizational principles also present in larger and more complicated nervous systems.
Picking out a complete ensemble can be almost impossible in more concentrated nervous systems, but cnidarians’ spread-out neurons make them particularly well suited for this line of research. “With nerve nets, you can see the activity of an entire population of neurons and make conclusions that are complete, accurate and permanent,” says Rafael Yuste, a neuroscientist at Columbia University. “There are no other neurons.” Yuste and other neuroscientists are now pairing newly developed tools for monitoring activity in cnidarian neurons with computational and machine learning tools to track behavior and to model the relationships between groups of neurons through their firing activity.
Though it’s still early days, new insights are revealing how ensembles control behavior, such as feeding behavior in the jellyfish Clytia hemisphaerica and bodily contraction and extension in the polyp Hydra vulgaris. Researchers are now starting to study how ensembles form and how groups of ensembles interact, efforts they hope will also inform our understanding of more complex nervous systems. Adrienne Fairhall, a theoretical neuroscientist at the University of Washington, believes the ability to comprehensively connect structure and function in these simple systems could reveal general principles of neural circuits, particularly how properties emerge in a distributed system of neurons. “How does that all work when there are no clear decision-making nodes?” she says. “Can we derive the rules by which this nervous system works, in this distributed-control manner?”
A somersaulting polyp
Hydra vulgaris, a cnidarian polyp species found in temperate and tropical freshwater environments, has fascinated biologists for hundreds of years — Hydra research documents date back to the 17th century. The animals reproduce via a peculiar mixture of sexual and asexual means and lack outward signs of aging, hinting at a sort of immortality. More recently, neuroscientists have started to take notice. Yuste first took an interest in the animal almost a decade ago at the suggestion of his mentor Sydney Brenner, a neurobiologist famous for developing the nematode Caenorhabditis elegans into a widely used model organism. Brenner held that a Hydra model could have a similar impact on science. He thought that Hydra’s uniformly distributed cnidarian nerve net, coupled with the organism’s near-transparency, offered real potential for scientists to make connections between neural ensemble activity and animal behavior. Yuste recalls Brenner suggesting Hydra as a perfect opportunity to really crack the neural code of a simple animal: “Instead of trying to break the neural code in the most complicated brain — the mammalian cortex — why not try the simplest ones?”
By clicking to watch this video, you agree to our privacy policy.
Hydra lead relatively sedentary lifestyles. One end of their body is attached to the floor of their aquatic environment, so they cannot swim freely through the water. However, they are notable among other polyp-like cnidarian species for their large set of distinct motor behaviors — in addition to pulsing individual tentacles, they can move by extending and contracting their trunk. In a striking act of acrobatics, they can also detach their base and bend their trunk to somersault along the floor.
When Yuste and collaborators first forayed into the world of Hydra, they lacked the massive arsenal of molecular and other tools developed for fruit flies and other model organisms over the previous decades. To leverage the animal’s unique nervous system, Yuste’s team developed calcium indicators to track neural activity in Hydra. “That enabled us for the first time to image the activity of every neuron in an animal,” Yuste says. When developing the cell targeting methods, Yuste’s collaborator, John Szymanski at Columbia, stumbled on the ability to target Hydra muscle tissue as well as neural tissue. “Before we knew it, we were imaging every muscle cell in the animal,” Yuste says. To tie neural and muscle activity to behavior, Yuste, Fairhall and colleagues developed computer vision algorithms to quantify the position of the animal’s squishy body as it moved. Together, those tools offered a path to developing one of the first full models describing the neural control of movement and behavior in an organism — and to realizing one of Brenner’s hoped-for outcomes of Hydra research.

The first step in developing the model was to observe the relationship between neural ensembles and repeatable behaviors. Recording spiking activity from neurons across the animal’s body, the researchers found 12 distinct, non-overlapping networks in the nerve net and discovered that three of them control distinct animal behaviors. The contraction burst, or CB, network activates muscles in the trunk, smooshing the animal down into a small nub and launching Hydra’s characteristic somersault. Two other networks, rhythmic potential networks RP1 and RP2, are named for the slow, oscillating nature of those ensembles’ firing patterns. Initial research found that RP1 and RP2 control bodily elongation, motions which stretch out Hydra’s trunk, and radial contractions, which Hydra uses to push material out from the cavity inside its body.
Researchers are still working to understand how the RP and other networks control behavior, but they have already identified key properties of the networks. Each Hydra neuron is a member of only one ensemble, indicating that those cells are involved in one specific motor behavior. The findings suggest that neural ensembles in Hydra are tightly fixed to specific animal behaviors, and that a neuron’s ensemble membership does not change between successive behaviors.
One of Hydra’s most striking features is its extraordinary capacity for regeneration. Not only can these polyps regrow lost body parts, they can completely reassemble themselves if they get split apart into a suspension of cells. Researchers are using this latter property to study how neuronal ensembles form. In a bizarre experiment to test the robustness of neuron-ensemble identities, Jonathan Lovas, one of Yuste’s graduate students, mapped out the ensembles in several Hydra specimens and then dissected the animals into small pieces. As the animals re-formed, their nerve nets rebuilt themselves to create the exact same ensembles — CB, RP1, RP2 and others — with each network controlling the same behaviors as before. However, individual neurons did not always join the same ensemble they had originally belonged to. The findings suggest that ensembles may form based on their activity patterns rather than genetic or other predetermined factors. The fact that Hydra ensembles form seemingly ad hoc from coactivating neurons may further indicate that ensembles could be governed by similar principles in higher organisms like rodents and humans.
Building outward to pull inward
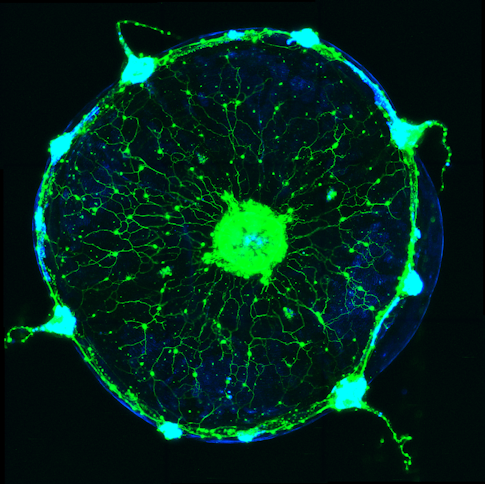
Jellyfish aren’t an obvious choice for studying emotion. But for David Anderson, a neuroscientist at the California Institute of Technology and an investigator with the Simons Collaboration on the Global Brain, they offer a unique opportunity to examine how an animal’s emotional state influences its broader brain function. Emotions, as we experience them, have a profound and long-lasting effect on our thoughts and behaviors. Anderson and others hypothesize that both human emotions and those of other animals manifest from persistent neurochemical states, which he calls emotional primitives, that influence how neurons function. Anderson’s lab searches for signs of these persistent mental states in neural activity. While most work in the lab has focused on mice and fruit flies, the researchers have developed tools for studying neural ensembles in the nerve nets of Clytia hemisphaerica, a species of jellyfish, as an additional venue for exploring the connections between persistent states, animal behavior and neural ensemble activity. Clytia’s ancient evolutionary origins and simple nerve nets make it a good candidate for searching out primitive forms of internal states.
By clicking to watch this video, you agree to our privacy policy.
By clicking to watch this video, you agree to our privacy policy.
When a jellyfish eats, it curls the edge of its body to bring food captured from a tentacle to its mouth. Anderson and collaborators found that wedge-shaped ensembles of neurons underlie this action, firing together as the animal snacks on shrimp. Each pizza-shaped wedge in the circular jellyfish controls muscles that fold an edge of the body inward to the animal’s mouth.
To study how ensembles interact, researchers gave Clytia food at different spots around the body’s outer edge. They found that only one wedge responded to food at a time — a new fold started only after a previous fold reopened — suggesting that ensembles inhibit each other.
Though scientists don’t yet know what underlies that inhibition, the findings hint at persistent interactions between the ensembles, bringing researchers one step closer to the internal states that Anderson is interested in.
Researchers don’t yet know how seemingly independent ensembles inhibit each other, but they are exploring this question with newly developed calcium imaging techniques that allow them to record from all of a jellyfish’s neurons. Their search will undoubtedly uncover many more functional ensembles controlling other jellyfish behaviors. The group’s ultimate goal is to create a whole-organism model connecting neural activity to behavior, similar to the one being developed by Yuste and Fairhall for Hydra.
While Yuste’s, Fairhall’s and Anderson’s groups have done a tremendous amount of work to develop these animal models, there is still much left to do before they can produce complete models of Clytia and Hydra. Anderson and collaborators have shown that wedge ensembles in Clytia clearly drive margin folding behaviors, but they still don’t understand the extent of those interactions. Similarly, Yuste and Fairhall have characterized only three of Hydra’s 12 identified ensemble networks. But Yuste is hopeful that these ensemble discoveries will eventually provide them with a powerful model of animal behavior. “This could be a revolution in neuroscience, even a paradigm shift,” he says. “These methods give us the chance to see all of a brain and understand exactly what it’s doing.”


