The RNA Detective

When Christopher Park was an undergraduate student, the human genome was starting to be sequenced with regularity. Researchers were beginning to study genetic variations in individuals and potential links to diseases. Learning about this research fascinated Park, and started him on a path to studying how disorders manifest from our genomes.
Today, Park specializes in RNA biology, which deals with ribonucleic acid, the single-stranded material that takes our DNA and turns it into the proteins cells use to grow and function. As a research scientist at the Flatiron Institute’s Center for Computational Biology (CCB), Park studies how RNA might be associated with neuropsychiatric conditions such as autism, schizophrenia, ADHD and bipolar disorders.
Before joining CCB, Park worked as a postdoctoral researcher at the Rockefeller University. He earned a doctorate in computer science with a focus on computational biology from Princeton University and a bachelor’s degree with dual majors in computer science and biochemistry from the University of Washington, Seattle.
The Simons Foundation sat down with Park to discuss his research and how it might lead to treating neuropsychiatric disorders in the future. The conversation has been edited for clarity.
What is the connection between neuropsychiatric conditions and RNA?
We know from studies of family history that there’s a large genetic component in neuropsychiatric conditions such as autism, schizophrenia and ADHD. But how that connection works at a biochemical level is still largely a mystery.
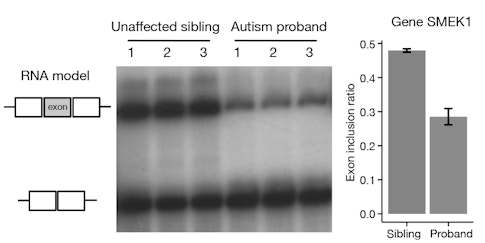
One of my goals is to try to see how mutations in RNA could play a role in causing these neuropsychiatric conditions. Many people are familiar with mutations in the DNA, but some of these mutations can also carry over into RNA, where they can disrupt how RNA binds to proteins. I’m trying to find what downstream biochemical mechanism might be altered when this protein-RNA binding process is disrupted. My research shows that an end process involving RNA, called post-transcriptional regulation, is very important.
What is post-transcriptional regulation?
In our DNA, each gene has to be turned on to be expressed. Once it’s expressed, its instructions can be copied into RNA to eventually create a protein. This process of copying DNA to RNA is called transcription.
Mutations in DNA can not only disrupt normal gene expression — they can also be copied in the RNA. At that point, there’s still a whole set of processes that occur before the RNA ultimately produces a protein. We call this post-transcriptional regulation.
Importantly, if DNA mutations make it into the RNA, those mutations can disrupt the binding of protein molecules to the RNA. This can prevent a cell from functioning normally or neurons from forming synapses, which could lead to certain neuropsychiatric conditions.
Mutations affecting gene expression have been a major focus of a lot of research, but post-transcriptional regulation has received less attention. My primary focus has been analyzing data with computational methods to shed light on the mechanisms that guide post-transcriptional regulation. I often take datasets from many experimental projects and combine them to answer new questions using computer models. This requires writing a lot of code for data analysis to help find patterns in large pools of data.
That work has been my focus for a while, but lately I’ve been transitioning to study the impacts of hormones on diseases, using similar computational techniques.
What kinds of impacts do hormones have?
Hormones — which are made by almost every organ — are deposited into the bloodstream, enabling organs that are very far apart to communicate with each other. This allows all the individual parts of our bodies to function together as a whole.
Research is beginning to find that hormones are important beyond just communication, in the context of neuropsychiatric conditions. Some of these conditions have specific onset ages: For example, schizophrenia first shows up around age 20. We think this is potentially linked to sex hormones, which generally spike to reach adult levels by age 18. Additionally, during childhood, boys are twice as likely as girls to be diagnosed with autism. In these cases, sex-hormone differences may play an important role. We know males have a spike of testosterone right around birth; and for all sexes, sex hormones typically surge during late teen years.
Interestingly, scientists are finding over and over again that there are these periods in brain development where the brain is very sensitive to change. And these periods might be delineated by some type of hormone surge. The time before the end of puberty is thought to be when the brain goes through dramatic change to prepare for adulthood. For example, experiments done on mice and flies have shown that blocking hormone signaling in the brain pre-puberty can have dramatic effects, such as on memory and mating behaviors, but the same blocking has little or no effect if done post-puberty.
Could this link with hormones ultimately lead to any medical treatments?
If there is a strong hormonal connection to a condition or disorder, that would be great, because hormones can be easily measured in the blood, making diagnosis easy. Additionally, there’s potential for a hormone to be administered therapeutically for treatment.
In fact, this has already been accomplished for diabetes. Diabetes is a disease when glucose levels are high because insulin, a hormone produced by the pancreas, isn’t being produced adequately. The therapy is injection of that hormone to help the body lower blood glucose levels.
For some neuropsychiatric conditions, there might be a similar treatment through the application of hormones. Even if the drug itself isn’t a hormone, maybe it is more effective at certain hormone levels. There’s a possibility that a drug might only work at very critical times during brain development. Hormone levels might guide us as to when is the best period to administer a drug. Obviously, we’re very far out from such treatment applications, but it’s something we’re starting to think about.
This Q&A is part of Flatiron Scientist Spotlight, a series in which the institute’s early-career researchers share their latest work and contributions to their respective fields.