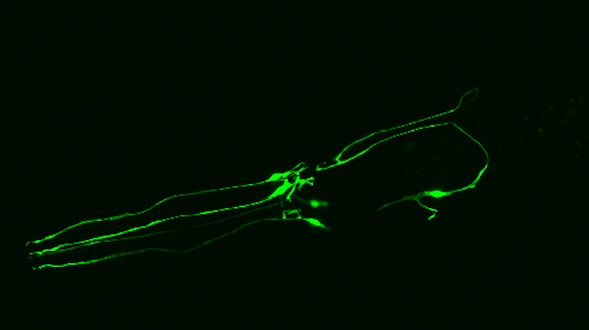
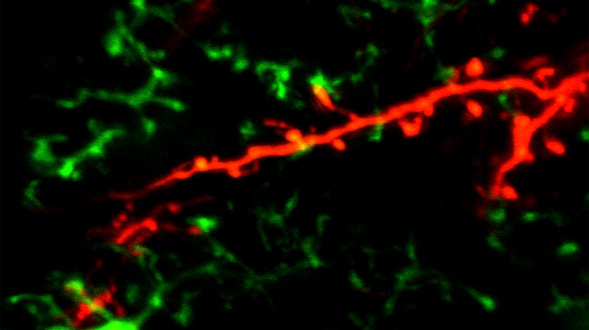
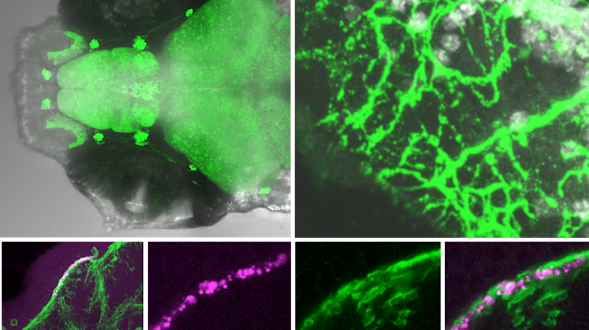
We will develop a centralized database to integrate data produced by the various Simons Collaboration on Plasticity and the Aging Brain projects. This type of database is essential because different species and individuals within a species follow different trajectories of brain aging, and researchers need a reference framework to link them. The framework we develop will include surface features of aging, such as life span, behavior, and gross morphological and functional changes, as well as deep features, such as shifts in cell states, emergence and attenuation of signaling pathways, and accumulation of underlying genetic and cellular damage. The overarching goal is to develop a database and interfaces that align knowledge about brain aging across model systems and humans.
Projects
As we age, our ability to learn and remember starts to decline, even in the absence of age-related dementia. We haven’t identified the critical molecular changes that occur in the aging brain or how these changes affect plasticity, the brain’s ability to rewire its connections. To develop interventions to slow or reverse this decline, scientists must first characterize these changes at the molecular and cellular levels and identify the key genes that regulate these processes in the brain. Both of these tasks are challenging. We will leverage the genetic similarities between humans and many different organisms, ranging from worms to fish to mice to nonhuman primates, to examine how these organisms’ ability to learn, a behavioral measure of plasticity, changes with age. This approach can identify a set of core hubs of plasticity in the aging brain that are conserved across species, as well as species-specific changes. We will assess how much these regulators are conserved across different cell types, across individuals of the same species and across species. The promising potential therapeutic targets are those that are shared across species, and using multiple model systems and integrating the information across species will yield information that is greater than the sum of the individual systems.
Our ability to learn, remember and make decisions changes with age. Older mice, for example, take longer to learn new tasks and don’t perform as well as their younger counterparts. We aim to discover how the neural circuits that support learning, attention and decision-making evolve with age and how these changes contribute to age-related changes in behavior. Our hypothesis is that many age-related differences in behavior and learning capacity arise not from neurodegeneration but from active remodeling of neural circuits. To test this hypothesis, we will track age-related changes in mice all the way from gene expression to complex behavior. We will use an automated system to characterize spontaneous behavior and to measure learning and expert performance on two complementary tasks that have already been thoroughly tested in young adult animals. Next, we will measure and manipulate neural activity in both cortical and subcortical structures to determine which brain areas mirror changes in behavior and when these changes are most prominent during the life span. We will also track the synaptic, cellular and wiring changes that occur in these brain areas during healthy aging. In addition to exploring neural changes, we will examine how glial cells contribute to age-related decline. Previous research in our group has shown that a set of genes active in glia may drive some of these changes. We will determine if silencing these genes can prevent age-related decline in learning and decision-making. Taken together, these approaches will greatly advance our understanding of the neural underpinnings of age-related changes in synapses, circuits and behavior.
Aging is accompanied by a striking reduction in deep sleep and sleep structure. Because sleep plays several essential roles in the brain, loss of sleep with age may contribute to cognitive decline — for example, sleep loss predicts individual memory ability in older adults. During sleep, glial cells and the lymphatic system help remove metabolic waste that accumulates in brain cells when we’re awake, reducing inflammation and restoring a healthy neural environment. Sleep is also crucial for regulating neural plasticity. During wakefulness, the size and number of synapses and overall excitability in the brain rises. Sleep restores the baseline and prevents the brain from becoming hyperexcitable.
It’s unclear exactly how age-related sleep decline disrupts cognitive function. We hypothesize that worsening sleep quality impairs waste removal, triggering low-grade neuroinflammation, slowing the birth of new brain cells and dampening synaptic plasticity. Our collaboration will identify how glial function during sleep changes with age in fish, mice and humans and how these changes influence inflammation and neural plasticity. We will determine if improving the quality of sleep and enhancing glial waste-removal systems makes animals more resilient to age-related cognitive decline. We will search for mechanisms that impair waste removal and determine how modulating these mechanisms influences plasticity, memory and the birth of new neurons. The results will highlight potential biomarkers that can be used to assess sleep-related damage with age, as well as targets for slowing sleep-related cognitive decline.
How does sleep sustain synaptic lipid function across life? The brain maintains broadly stable circuit function, reflected in precise tuning of synaptic connections and neuronal morphology, over days, months and years. As most neurons in the central nervous system survive throughout adult life, the homeostatic processes that maintain brain function must be near-perfect over long timescales. At the same time, aging almost inevitably diminishes cognitive function, likely reflecting how small imbalances in these homeostatic processes gradually accumulate to diminish the function of individual cells and specific circuits. We hypothesize that sleep is a recurring state with a fundamental homeostatic function for neuronal integrity, and thus a key regulator of the effects of aging. Sleep integrity is paramount for memory and cognition, and sleep fragmentation in normal aging is thought to be responsible for decreased cognitive and memory performance. Further, the functions of sleep in regulating cytoplasmic membrane composition, synaptic strengthening and neurite pruning are highly conserved across species and have been demonstrated in fly, zebrafish, mouse and humans. Moreover, while the proteins involved in synaptic plasticity and neuronal biology has been extensively studied, homeostatic processes that shape the specialized lipid membranes of neurons are largely unknown. The goal of this proposal is to understand the sleep-dependent molecular mechanisms that underpin how neuronal membrane function is maintained over long timescales.
As we age, most individuals will experience normal declines in cognitive abilities, including memory. These changes are a natural part of aging and are not associated with pathological causes of memory impairment or cognitive decline. However, rates of cognitive aging differ among individuals, with some people exhibiting high levels of cognitive function even late in life — a phenomenon known as “cognitive resilience,” where cognitive function is better than predicted based on a person’s chronological age. While the causes of age-related cognitive decline are largely unknown, heritability estimates indicate there is a strong influence of yet-to-be-identified genetic factors in age-related cognitive resilience. Identification of these factors represents an opportunity to develop new therapeutic approaches to enhance and preserve cognitive function in aging individuals. As the population ages, the need for such interventions becomes increasingly urgent, but efforts to understand the mechanisms governing cognitive resilience in humans has been limited by a lack of access to brain tissue from successful aging humans as well as longitudinal measures of cognitive function across the lifespan. Model organisms such as mice represent a powerful tool to interrogate the underlying genetic and molecular mechanisms governing cognitive resilience, but most models fail to capture the natural genetic variation existing in human populations, limiting translational applicability.
Advances in disease prevention and therapeutics have enabled humans to live longer than ever before. We now face the challenge of increasing health span in a comparable manner. How can we help people maintain the normal mental function needed to support multifaceted, independent lifestyles for as long as possible? The cognitive changes that develop with normal advanced aging are poorly understood, and we currently lack effective therapies to slow decline. We know that age-related changes in the brain occur at the molecular, circuit and behavioral levels, so we must investigate each of these scales and understand how changes at one level relate to changes at others. We aim to understand the links between molecular, circuit and behavioral alterations that occur with age, with a longer-term goal of developing effective treatments that will help maintain cognitive function during aging.
As we age, our bodies and brains decline. But in some individuals, even into the eighth and ninth decades, memory and cognitive flexibility remain sharp. A challenge is finding these individuals and harnessing the factors that have allowed them to age so successfully, and also to disentangle differences in how life trajectories start from and how they change with advanced aging. Our project's goal is to characterize brain structure and function in relation to maintained cognition and memory, explore biological and lifestyle factors that support maintenance, and develop novel approaches to characterize brain aging trajectories within individuals over short time periods. We aspire to provide a conceptual foundation and empirical strategies to explore factors that maintain and promote brain health.
The world’s population is rapidly aging. Age-related cognitive decline is a major biomedical challenge with no effective medical treatments. However, aging affects men and women differently on average — most women live longer than men worldwide and in many populations appear to experience less cognitive decline and to lose less brain tissue over time. However, many women undergo dramatic loss of sex hormones compared to men, which may make their decline different from men’s. Sex chromosomes and sex hormones are likely involved, but the exact mechanisms that underlie these fundamental differences in brain aging and cognitive decline — both between the sexes and within each sex — remain largely unknown. Understanding what makes one sex more vulnerable or resilient to aging will reveal novel targets for treatment that could benefit everyone.
Cognitive function declines with age, reflecting a complex and interrelated set of changes in neurons, neural stem cells, glia and vascular cells. This complexity presents a formidable challenge to neuroscientists studying the aging brain. However, a series of surprising observations has provided an exciting path forward. We have discovered that blood contains proteins that can restore some age-related cognitive decline. For example, giving old mice factors found in young blood can enhance the birth of new neurons, which typically slows with age. Blood proteins from younger animals can also restore declining blood vessel function in older animals, improving blood flow and the delivery of key nutrients to brain cells.
We have identified several different components in blood that have this effect, each of which appears to act differently. Certain factors appear to cross the blood-brain barrier and bind directly to receptors on neural and glial cells. Other factors may act on vascular cells, boosting neural function via better blood flow or by increasing the vascular system’s secretion of neuroactive factors.