
Rethinking the Spectrum: Three Things We Thought Were True About Autism
 In 2003, when the Simons Foundation Autism Research Initiative (SFARI) launched, little was known about the biology of autism. Studies of twins with autism had highlighted a strong genetic component, but how this genetic risk was transmitted and the mechanisms by which genetic changes impact development were unknown. For SFARI, understanding autism genetics was not only a promising area of investigation, it was the scientific priority to advance the field.
In 2003, when the Simons Foundation Autism Research Initiative (SFARI) launched, little was known about the biology of autism. Studies of twins with autism had highlighted a strong genetic component, but how this genetic risk was transmitted and the mechanisms by which genetic changes impact development were unknown. For SFARI, understanding autism genetics was not only a promising area of investigation, it was the scientific priority to advance the field.
Seventeen years later, new insights about autism have sprung up, many of which have compelled scientists to reevaluate how they think about the condition. In particular, three ideas about autism have turned out to be different, or more complex in nature, in light of new knowledge.
Original idea 1: A few genes would contribute to a significant number of autism cases.
The year SFARI launched coincided with the first identification of genes linked to autism. Additional genetic findings soon followed, and within a few years, about 20 individual genes and several copy number variants (CNVs) — large chunks of the genome containing multiple genes — had been implicated in the condition.
Although these discoveries represented tremendous contributions to the field, scientists were, in fact, just scraping the surface of the complex autism genetic landscape. The number of autism risk genes and CNVs turned out to be much higher than earlier studies had suggested.
The SFARI-funded Simons Simplex Collection (SSC) has greatly facilitated this process of gene discovery. The SSC is a repository of genetic and phenotypic data from more than 2,000 individuals with autism, and their parents and unaffected siblings. SSC data have now been used in more than 200 research articles, and scientists have been able to identify more than 150 genes and many CNVs linked to autism risk with high levels of statistical significance.
A second SFARI-funded project is currently scaling up the SSC’s initial efforts. Called Simons Foundation Powering Autism Research (SPARK), this initiative, which began in 2016, aims to establish the largest autism research cohort to date, including 50,000 individuals with autism and their family members. Preliminary analyses of the first 500 families enrolled in this initiative confirmed many previously known autism risk genes and found several novel ones, showing that SPARK is well positioned to expand what we know about autism genetic risk factors.
“We believe that this year findings from SPARK will likely double the number of genes currently known to be associated with risk for autism,” says Wendy Chung, SFARI director of clinical research and Kennedy Family Professor of Pediatrics in Medicine at Columbia University.
The discovery of myriad genetic risk factors has been key to understanding that several distinct subtypes of autism may exist that can be defined by their different genetics. It is also becoming clearer that different forms of autism share convergent biological mechanisms, such as those affecting early brain development and communication between neurons. This is important not only to provide a finer-grained description of the condition, but also to develop targeted treatment strategies.
Original idea 2: Neurons are the primary brain cell type affected in autism.
Many autism-linked genes regulate the function of neurons, the most widely studied cells of the brain. Neurons, however, are not the only type of brain cell affected in autism. In particular, the brain’s resident immune cells, known as microglia, are susceptible. These cells protect the brain from insults caused by infection and inflammation and play critical roles as synapses mature and brain cells wire up during development.
Several SFARI investigators, including John Allman of the California Institute of Technology, Dan Arking of the Johns Hopkins School of Medicine and Arnold Kriegstein of the University of California, San Francisco, have found changes in the number, shape and gene activity of microglia in postmortem brain tissue from individuals with autism. Whether these alterations reflect a primary cause or a secondary effect of autism, however, remains unclear.
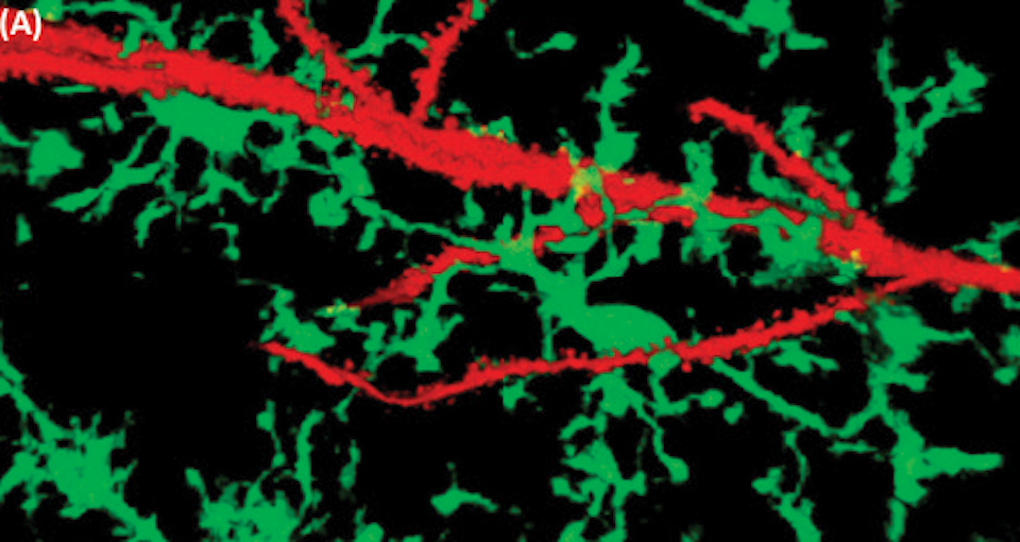
A number of other SFARI-funded projects are trying to better understand the function of microglia in autism and related conditions. For instance, SFARI investigator Beth Stevens of Boston Children’s Hospital has demonstrated that, in healthy mice, microglia help to shape neuronal connections by removing those that are less active. This pruning of neuronal connections, however, was excessive in a mouse model of Rett syndrome — a severe neurodevelopmental condition caused by mutations in the MECP2 gene. These findings led Stevens to argue that microglia play a role in the neuropathology of Rett syndrome. They also formed the foundation for new studies on microglia in other mouse models of neurodevelopmental conditions, including those with autism-relevant genetic and environmental perturbations, which she is now conducting in collaboration with SFARI investigator Steven McCarroll of Harvard University.
“Microglia have been overlooked for a very long time, but it’s now clear that we need to better appreciate the interplay between neurons and non-neuronal cells if we want to understand brain functions in health and disease, as well as development,” Stevens says.
Original idea 3: Brain changes occur in the first few years of a child’s life.
Autism traits are usually identified within the first few years of life. As a consequence, researchers thought that brain changes associated with the condition would also occur in the first years after birth. Brain differences may indeed become evident in early childhood, but scientists have come to realize that in children later diagnosed with autism, things start progressing down an atypical developmental path during gestation, while the brain is still forming.
Multiple finely tuned and precisely timed mechanisms are responsible for brain development in utero, so many possible points of disruption exist in this choreographed process. SFARI investigators Matthew State of the University of California, San Francisco, Bernie Devlin of the University of Pittsburgh and Nenad Sestan of Yale University showed that autism-associated genes specifically regulate brain developmental processes that occur during the first 10 to 24 weeks of gestation, suggesting that early midfetal life is a critical window for the condition.
“We may not know how all the factors involved in autism come together, but we’re starting to learn when and where many of those changes are likely to occur,” State says.
The finding that brain changes associated with autism occur prenatally implies that early behavioral signs or biological markers may be detectable soon after birth. This is important, because the earlier the diagnosis, the better the chance for effective therapy. Accordingly, SFARI has funded research to identify early behavioral changes and predictive biomarkers.
Promising steps in this direction have been made by Ami Klin of Emory University, one of the first investigators to receive a SFARI grant. He found that, between the ages of 2 and 6 months, children later diagnosed with autism show decreased interest in looking at another person’s eyes, compared with children who develop typically. Gaze and eye contact are among the first social behaviors in an infant’s life; thus, it is conceivable how alterations in these initial signs of communication may have profound effects on establishing relationships with others.
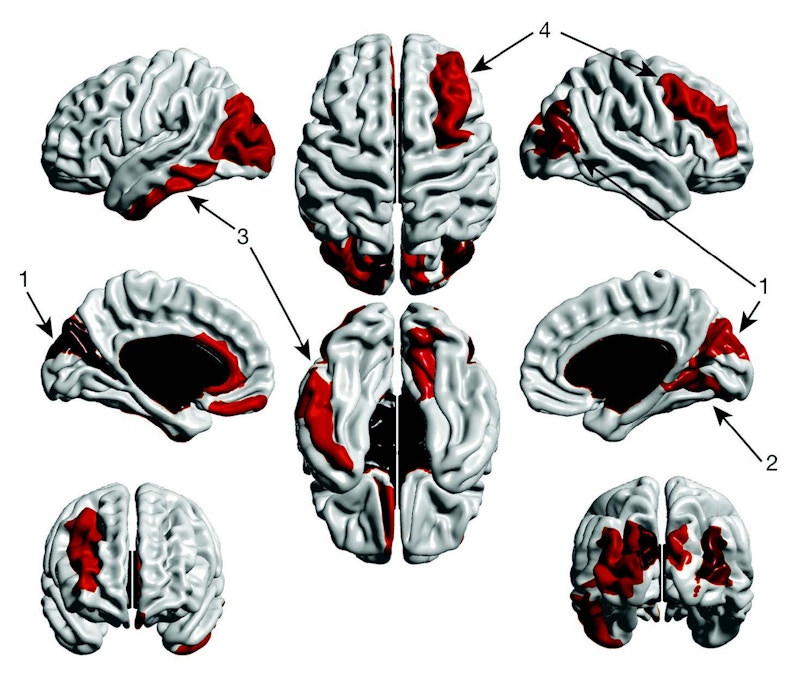
The observation of changes in these early behavioral signals also suggests the presence of underlying brain changes soon after birth, which may be potentially used as biomarkers of autism. Using brain-imaging techniques, SFARI investigator Joseph Piven of the University of North Carolina at Chapel Hill examined high-risk infants (i.e., siblings of children with autism) and found that, as early as 6 to 12 months, the cortical surface of the brain was larger in those infants who were later diagnosed with autism, compared with those without the condition. When plugged into a machine-learning algorithm, this early cortical hyperexpansion was the brain measure that best predicted which infants would later go on to be diagnosed with autism.
These findings suggest that specific, early changes in brain size may predict autism even before behavioral traits become apparent and a clinical diagnosis is made. One future challenge will be to determine whether and how potential autism biomarkers can be incorporated into clinical practice to accelerate the diagnostic process, track an individual’s response to treatment, and ultimately help to improve outcomes for individuals with autism and their families.
