From Ring to Keyhole: Modeling How Tissues Mold the Fruit Fly’s Gut
Researchers at the Flatiron Institute’s Center for Computational Biology and their collaborators from Princeton University and the Max Planck Institute in Dresden, Germany, use experimental and computational models to demonstrate how mechanical forces from surrounding tissues can shape the fruit fly gut. The results lay the groundwork for understanding how organs and tissues take shape in embryos across many species.
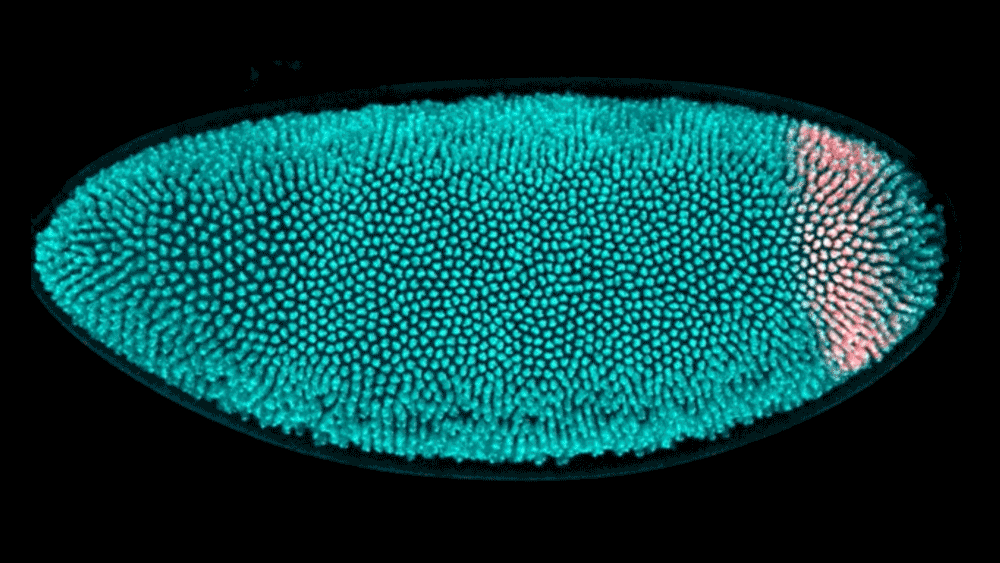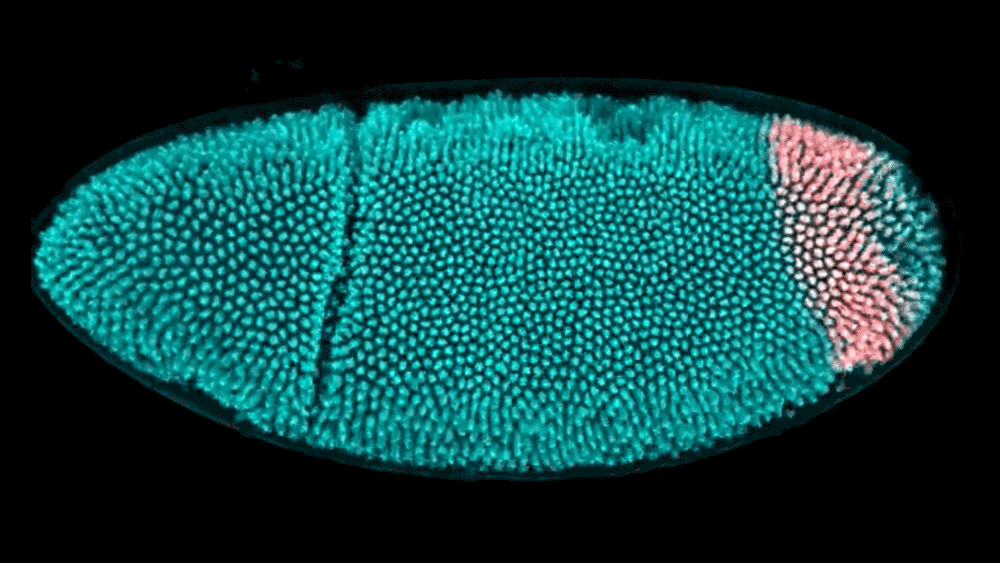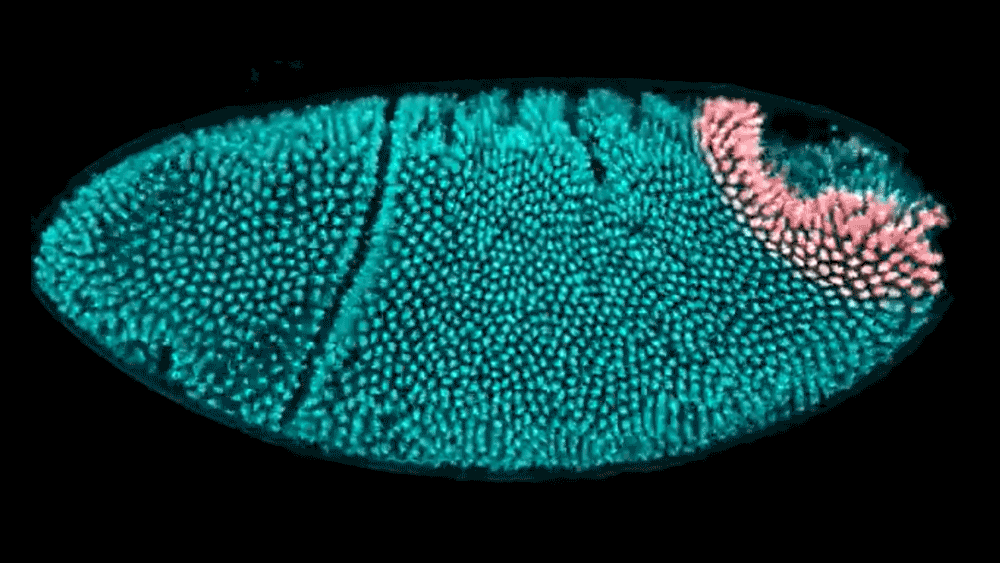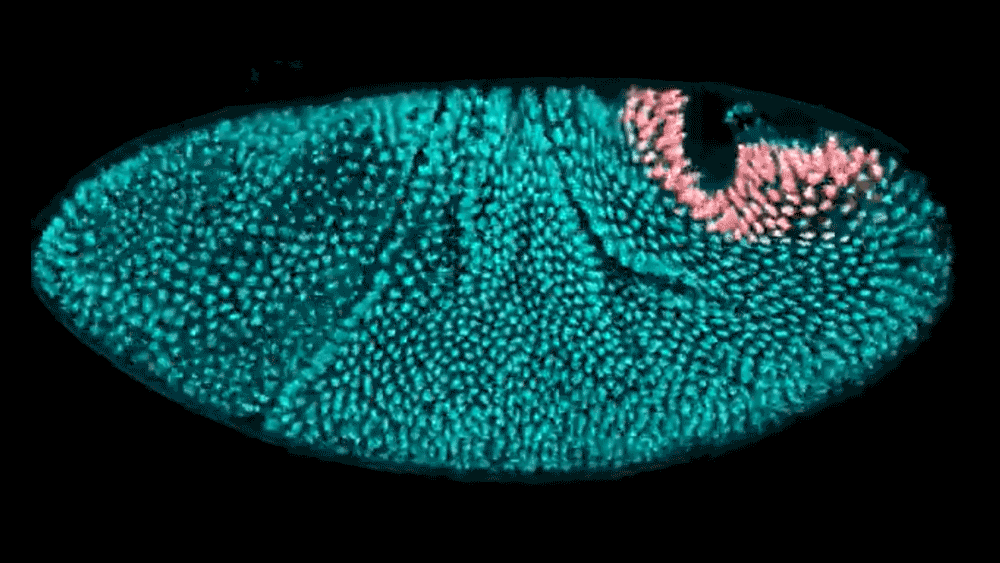
The shape of a kidney is so distinct that a bean is named after it. The liver forms a broad wedge tucked beneath the ribs. And the heart bears almost no resemblance to the universal symbol. But how do these organs acquire their signature shapes? Using cutting-edge computational modeling, researchers at the Simons Foundation’s Flatiron Institute and their collaborators have developed a better understanding of how neighboring tissues mold the shape of developing organs and tissues.
The researchers examined the embryo of a well-studied organism — the fruit fly — to track how its hindgut develops. The work, reported in Proceedings of the National Academy of Sciences, shows that the way tissues push on each other is critical to creating the hindgut’s complex shape. Based on these experimental findings, the researchers created a computational model to show exactly how the hindgut morphs from a ring shape to a keyhole shape. With these tools, researchers now have a better way to study large systems of tissues as they develop.
“Our findings suggest that the hindgut’s complex shape can be explained by simple mechanical principles,” says Daniel Alber, a graduate student at Princeton University working with Stanislav Shvartsman, a senior research scientist at the Flatiron Institute’s Center for Computational Biology (CCB) and a Princeton professor.
The work also establishes the fruit fly hindgut as a fitting model for studying complex tissue development.
“There is already a huge body of literature about how the fruit fly develops, more so than almost any other system, which is important groundwork to have if you want to examine multiple tissues at once,” says Alber. “Our work demonstrates that this organism can provide important insights into a complicated process.”
Alber and Shvartsman co-authored the study with the CCB’s Alexandre Jacinto; Shiheng Zhao and Pierre Haas from the Max Planck Institute for the Physics of Complex Systems and the Max Planck Institute of Molecular Cell Biology and Genetics; and Eric Wieschaus of Princeton.
Shape-Shifting Tissues
Tissues achieve their final form by undergoing a process called morphogenesis. There are two main types of morphogenesis: active and passive. In active morphogenesis, biological processes generate forces within a tissue, pushing at its boundaries from the inside to shape it. Passive morphogenesis occurs when external mechanical forces, such as pressure from bumping into neighboring tissues, mold the tissue from the outside.
Active morphogenesis is easier to study than passive morphogenesis because it all takes place within a single tissue. To fully capture passive morphogenesis, scientists must observe an entire system of tissues, which is far more difficult and requires examination of an already well-understood organism. Fortunately, the team found a suitable candidate in the fruit fly hindgut, a well-characterized ring of tissue in the early fly embryo that goes from a circular ring to a complex, asymmetrical shape in just 15 minutes.
Using light sheet microscopy, in which an ultra-thin laser illuminates a sample, the team recorded footage of developing fruit fly embryos at 25 frames per second. Thanks to the Flatiron Institute’s powerful computational resources, the scientists could leverage cell tracking algorithms to follow each cell in the hindgut ring, precisely mapping how it changes shape. Based on these data, the team built a computational model showing that neighboring tissues undergoing their own active morphogenesis essentially bump into the hindgut tissue, causing it to morph from a ring into a triangular keyhole shape.
In short, the formation of the hindgut’s complex shape appears to be largely due to passive morphogenesis, a surprising result, as such dramatic development could be expected to require active morphogenesis. More importantly, the team stresses, the work demonstrates the power of the model for studying a phenomenon that’s so difficult to capture.
“What we’re most excited about isn’t the fruit fly itself, but the potential of the model to inform our knowledge about other systems,” says Alber. “We hope this paper will encourage others interested in passive morphogenesis to study it in the fruit fly.”
A Deeper Dive Into Development
This work has exciting implications for understanding developmental biology as well as engineering tissues to treat disease or injury.
“To explain how an embryo becomes an organism, you have to explain how everything achieves its shape, not just parts of it,” says Alber. “This work helps fill in that gap of what happens to the tissues that aren’t changing shape on their own. And if your goal is to engineer a tissue or organ that needs to have a certain shape, this work can help you to predict how surrounding tissues will influence that shape and plan for it.”
Next, the team will further explore the processes that drive passive morphogenesis and establish a shared vocabulary to describe the phenomenon.
“Future studies will aim to uncover the molecular and cellular mechanisms that control this passive morphogenesis and its implications for tissue development and disease,” says Shvartsman.
“It’s a very rich dataset, and we’re really just at the tip of the iceberg of unpacking what’s happening,” says Alber. “And with all these 3D views of structures, it can be hard to know what you’re looking at. In follow-ups, we’d like to find better ways to describe how things deform in 3D so that there’s a shared language around it.”


