
The Challenges of Studying Elderly Animals
Back when Saul Villeda first launched his lab at the University of California, San Francisco, staff at the animal care facility alerted him to a problem with his mice — some of them had telltale signs of illness. According to the facility’s protocols, those animals would need treatment if they were to be kept alive. But the mice weren’t sick. They were just old.
Villeda studies how proteins and other molecules found in blood can influence aging in the brain. His research relies on mice that are 24 months old, roughly equivalent to a 60- to 70-year-old human, and thus far older than the vast majority of mice used in biological research. Indeed, animal care technicians were so unused to taking care of older animals that Villeda had to educate them about the physical and behavioral changes to expect, from sensitive skin and a more disheveled appearance to increased aggression. “These animals are going to lose hair,” says Villeda, an investigator with the Simons Collaboration on Plasticity and the Aging Brain. “It’s normal aging. This is fine.”
Researchers who are new to studying aging often confront new challenges when they begin working with animals nearing the end of their lives. Be they worms, mice or humans, these animals are in some stage of physical decline. They may be losing their hearing and eyesight or dealing with muscle loss and arthritis, problems that may cause animals to struggle with tasks used to assess their cognitive abilities. Or they may not live long enough to be included in the experiment at all. Everything from behavior to gene expression is more variable than it would be in a group of younger animals. And because aging proceeds differently in different individuals, it’s usually impossible to predict which animals will develop problems that will interfere with a planned study.
Many of the practical issues presented by elderly animals are manageable with some planning and adaptation. But researchers who study aging can’t shy away from a certain amount of complexity. While some aspects of biology unfold in a predictable, stereotyped manner, aging is stochastic, says Coleen Murphy, a biologist at Princeton University and SCPAB’s director. “The job of an aging researcher is a little tougher in that regard, because you don’t know, in a particular animal, when things are going to start to fall apart,” she says.
What’s more, aging affects both mind and body, and both genetic and environmental factors influence how it progresses and how its effects intersect. Amid all of this, researchers must find ways to discern what’s fundamental about aging and what steers the course of an individual’s decline. That, they say, will be critical for devising ways to preserve cognitive function, both in the course of healthy aging and in the midst of disease.
Whole-Body Aging
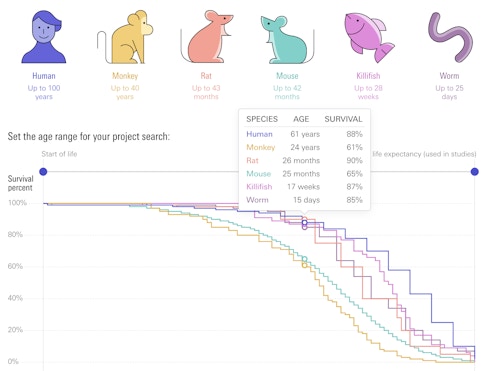
It’s difficult to define what ‘old’ is in a mouse or a monkey or, for that matter, a human. Villeda chose to study 24-month-old mice because at that age, about half the animals have consistent impairments. “And they’re not so old that they’re frail and can no longer physically do the task itself,” he says. (To compare the life spans of different animals used in SCPAB projects, check out this infographic.)
Still, Villeda and others find that by the time their animals reach the age at which cognitive problems are expected, some individuals do have impairments that can skew experimental results. Fortunately, such problems are usually easy to detect. Villeda says his lab screens animals for physical fitness prior to including them in their experiments, monitoring their activity to be sure they are capable of navigating a water maze, for example. For tasks that require animals to learn to associate particular sights or sounds with a reward, researchers may also need to screen for sensory deficits: An older animal may not be able to hear auditory cues, or may only respond to visual cues if they have a high-contrast design. Old animals are also more likely to die mid-experiment, so researchers need to start with more animals than they would otherwise.
Rozalyn Anderson, an SCPAB investigator at the University of Wisconsin-Madison who studies how a restricted-calorie diet slows aging in both mice and primates, agrees that many age-related deficits are easy to spot — aging animals are so closely monitored that caregivers usually know which individuals are struggling with physical ailments. “If there’s an outlier, you have a pretty good idea of the source of the problem,” she says.
But some issues are less clear. “Older mice tend to just sit there,” says Anderson. “They don’t want to do stuff.” It can be hard to interpret what it means when an old animal doesn’t engage. There’s evidence that the motivation to learn new things can decline later in life, in both humans and animals. So if a particular mouse doesn’t explore as much as expected, does that mean it has forgotten where to look for a reward? Or has it just lost interest entirely? Scientists can avoid this issue by selecting tasks motivated by the animals’ most innate instincts, Villeda says, such as a water maze that a mouse must navigate to access a safe space. “We use natural, innate motivation for survival,” he explains. “So the motivation is great.”
Randy Buckner, a neuroscientist at Harvard University and an SCPAB investigator, says researchers who study brain aging in humans also have to be mindful of physical issues. “One does have to worry about burden, always, with human participants,” he says. “Is a study too long? Is it too boring?” Buckner uses neuroimaging to study how the brain’s organization changes during both normal aging and disease. The older subjects in his studies are typically less comfortable in an MRI scanner than their younger counterparts, so researchers make sure to keep things short. Participants in their 20s might be expected to spend two hours in the scanner, but for seniors, the team limits each round of imaging to under an hour.
Fortunately, Buckner says, seniors tend to be eager to contribute, and most are open to repeat visits to the clinic. “It is a very strongly engaged community of volunteers,” he says. “As we age, I think many of us gravitate toward helping the community.” What’s more, he adds, those volunteers have been through medical procedures before, they’ve lived full lives with diverse experiences, and they’re tough. “Young man,” one study participant told him, “I was a fighter pilot in World War II. I think I can get through your study.”

Dealing With Diversity
Physical decline isn’t the only change that characterizes aging. As animals grow old, behavior and other factors become more variable, a pattern that presents both challenge and opportunity.
Roundworms, whose life expectancy is just a few weeks, really only behave predictably during their first week of adulthood, Murphy says. After that, individual behavior begins to diverge as cognitive and physical functions deteriorate. “This increased variation is part of aging. One animal might lose their cognitive ability really quickly, and others might maintain it a long time,” Murphy says.
At the same time, she says, there’s likely to be useful information hidden within this variation. For example, with collaborators Hong Gil Nam and Seung-Jae Lee in South Korea, she and her team found that worms’ maximum velocity at middle age (which approaches half a millimeter per second for the speediest worms) is a surprisingly reliable indicator of individual longevity. Murphy says her team tracked a host of measures in their middle-aged worms, and none correlated with longevity as well as maximum velocity. “It’s like you took a bunch of 40-year-olds and lined them up and made them do a hundred-meter dash, and then said, ‘OK, you’re going to live a long time and you are not,’” she says.
She acknowledges it’s difficult to find out which parameters will be most useful in predicting aging in other animals, but she thinks it’s worth the search. “It will be really interesting to see if [researchers working with other models] can pull that same kind of information out of their data in middle-aged animals,” she says.
Age-related variability isn’t limited to behavior. Virtually all types of data, from gene expression to neural anatomy, show a greater spread in groups of old animals than they do in younger ones, which can make data interpretation more difficult. One solution is simply to scale up: Study more animals to get a fuller picture of this variability and a more reliable measure of population trends. “For researchers who have never worked with old animals before, it would be really easy to vastly underestimate the number of animals you need to study when you’re trying to get out something that’s highly variable,” Murphy says. That’s doable for researchers who study worms or fish, but more costly and logistically challenging for scientists who work with rodents or larger animal models. And for primate studies, it’s simply not practical.
Indeed, age-related variability is particularly challenging to study in primates. Anderson points out that the monkeys she studies are genetically diverse, meaning she is grappling with both genetic and age-related variability. “You’re dealing with that double hit on the variance by the time you get to old age,” she says. Her team tracks many different parameters, from body weight to tissue-specific molecular profiles, that shift with age. Each of these can vary significantly between individuals. But by following them over a lifetime, with each animal serving as its own baseline, the team can see patterns emerge. “That gives you a better way to get after the data,” she says. “We tend not to focus on one particular measure, but more how that measure relates to other measures, and then how those things together might inform about aging.”
Adding to this complexity, different parts of the body can age at different rates. (For more on this, see “Molecular Clocks Offer New Insight Into Aging.”) The Boston Children’s Hospital neuroscientist Beth Stevens, a newcomer to the study of normal brain aging, studies the role of the immune system in the brain and how it influences the formation and elimination of synapses. Part of the challenge in understanding these processes, she says, is that they do not originate exclusively in the brain. Aging itself seems to reprogram some aspects of immune signaling, and these changes, both within the brain’s resident immune cells and elsewhere in the body, likely contribute to cognitive decline. Layered on top of this are the effects of injury and inflammation, which become increasingly common as we age. “If another organ is starting to malfunction and that was indirectly leading to these changes, that could also introduce a lot of variability,” Stevens says.
Making sense of all this variance can be complicated — but the fact that aging proceeds differently in every individual is what draws many scientists into aging research in the first place. Most hope that if they can sort out the causes of that variability, they will be able to identify which individuals are at the greatest risk of cognitive decline and find ways to intervene.
It’s easy to be frustrated by the vast differences in how the human brain ages, Buckner acknowledges: It’s not just difficult to predict the course of cognitive decline for any individual; it’s hard to even define what normal brain aging is. “But at the same time, it is the opportunity,” he says. “Because there is so much variability, that creates the opportunity to understand why.”


